Why choose CODVO

Outcomes over pilots
We land fast with focused pilots, then scale with evidence packs (model cards, validation protocols, traceability).

Regulatory-grade by design
Our SDLC aligns to ISO 14971 risk, IEC 62304 software lifecycle, IEC 82304-1 health software safety, and 21 CFR Part 11 documentation.

Interop at scale
FHIR/HL7/DICOM pipelines with de-identification, provenance, and TEFCA-aware exchange.

Edge to cloud
Telemetry and video analytics at the device/OR edge with secure update/rollback and post-market monitoring.

Enterprise velocity
AWS co-sell ready partner with repeatable blueprints that accelerate workload adoption.
Positioning note: We complement hyperscalers. You bring the cloud and EMR/EHR; we bring regulated delivery, governance, and workflow change that make AI stick.
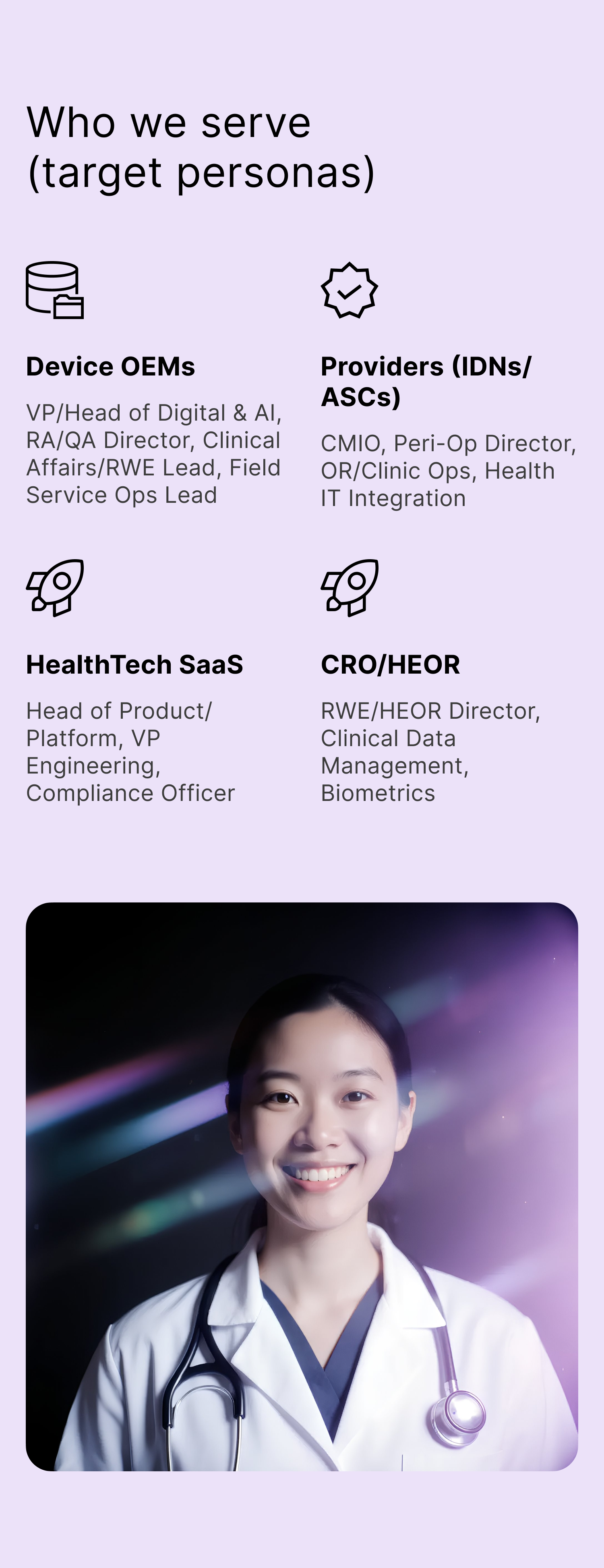
Industry Segments


Our focus pillars in Medtech practice



Data Engineering & Integration
Turn fragmented clinical and device data into governed, analytics-ready assets.
- FHIR/HL7/DICOM ingestion, harmonization, and lineage
- HIPAA-aligned PHI handling, de-identification, access policies
- Lakehouse on Databricks/Azure/AWS; CDC & near-real-time feeds
- Provenance/audit for RWE and safety reporting
Applied AI & Analytics
Deliver clinician-grade experiences and measurable operations impact.
- Ambient scribing (clinic/OR/telehealth), structured note generation
- Medical NLP, summarization, coding assist (CPT/ICD)
- Throughput, utilization, and quality analytics; predictive ops
- Explainability dashboards and model performance monitoring

Regulatory AI & Governance
Make AI "submission-ready" with evidence and traceability.
- Design controls & traceability matrices
- Model cards, validation protocols, and reports (WER, precision/recall, latency)
- 21 CFR Part 11 documentation automation & e-sign trails
- Post-market performance monitoring (PCCP-ready mindset)

Edge & Device Intelligence
Bring intelligence to the OR and the field—safely.
- Multi-camera OR/video pipelines; device telemetry ingestion
- On-device/near-edge inference, quantization, and optimization
- Secure OTA/update with rollback, SBOM & vulnerability workflows
- Predictive maintenance and field service enablement

Advisory & Enablement
Navigate strategy, compliance, and organizational change.
- SaMD/AI roadmap & risk posture reviews
- EHR/Ecosystem integration plans and partner selection
- Clinical workflow change-management and training
Proven Patterns
1
Ambient Scribe Accelerator
Where it fits
Ambulatory clinics, ASCs, telehealth, peri-op documentation
What it does
Captures encounters; generates structured notes; routes through HITL review
What's included
Accuracy/latency dashboards, audit logs, EHR integration ladder, PHI vault
2
RWE Fabric on FHIR/OMOP
Where it fits
OEM post-market safety, HEOR/clinical affairs, hospital quality teams
What it does
Harmonises multi-source data; builds evidence dashboards; supports PMS/PMCF analytics
What's included
De-ID pipeline, lineage/provenance, submission-friendly exports
3
Edge/Device Telemetry → Predictive Service
Where it fits
OR/robotics video, imaging devices, connected capital equipment
What it does
Detects anomalies; projects MTBF; reduces truck-rolls; informs service SLAs
What's included
Secure OTA/rollback, SBOM workflows, model governance hooks
Results that matter (select outcomes)
35-45%
Documentation time reduction
≤2 edits/note in production with HITL; p95 latency targets met for web/mobile
Clinical documentation
35–45% documentation time reduction; ≤2 edits/note in production with HITL; p95 latency targets met for web/mobile.
10-30
Concurrent streams
Multi-feed video ingest with anomaly detection improving MTBF and lowering service dispatch
OR & device operations
Multi-feed video ingest (10–30 concurrent streams), anomaly detection improving MTBF and lowering service dispatch.
RWE & safety
Harmonised datasets with provenance powering faster safety signal review and evidence generation.
(Metrics are representative outcomes from pilots and production programmes; detailed case studies available on request.)
Credibility & compliance
Regulatory
alignment
alignment
- ISO 14971 risk management, IEC 62304 software lifecycle, IEC 82304-1 health software, 21 CFR Part 11 records/signatures
- Usability/human-factors integration (IEC 62366) where applicable
- Cybersecurity posture with SBOM, threat modelling, vulnerability management, and incident playbooks
Evidence kit (delivered as part of projects)
- Model Card → data sources, performance, limitations
- Validation Protocol → test design, acceptance criteria
- Validation Reports → WER, precision/recall, latency, bias checks
- Traceability Matrix → requirements ↔ tests ↔ risks ↔ evidence
- CFR-11 Document Pack → versioned approvals and e-sign logs
Selected engagements & proof points

Surgical Scribe for U.S. ASCs
Scope: Procedure note generation with domain vocabularies and HITL QA
Impact: Time-to-note down; edits per note; cost/encounter visibility; roadmap for coding assist

Digital Polyphonic Development (MedTech OEM)
Scope: Edge video + device telemetry, cloud analytics, and DevSecOps/Governance
Impact: Faster cycle from event detection to action; service operations KPIs; submission-friendly audit trails

OR Efficiency Analysis (Edge + Vision)
Scope: Multi-camera pipeline, event detection, throughput analytics
Impact: Improved OR utilisation; decision support for staffing and scheduling


Platforms & Partners
Cloud & Data
Databricks (Delta/Unity/MLflow), Microsoft Azure (Health Data Services), AWS (HealthLake/Bedrock), Google Cloud (Healthcare API)
EHR/Interop
SMART on FHIR, FHIR Bulk APIs, HL7, DICOM gateways
Quality & DevSecOps
Git-native traceability, SBOM/CycloneDX, OpenTelemetry

Thought leadership & resources
Whitepaper: Audit-Ready AI in MedTech—From Model Card to Submission Pack
Tech Note: Ambient Scribe—Accuracy, Workflow & Cost Benchmarks
Playbook: RWE Fabric on FHIR/OMOP
Guide: Edge Telemetry to Predictive Service—A Reference Pattern
Get Started
Ready to build audit-ready MedTech solutions?